Welcome to the realm of the Law of Conservation of Mass, where the fundamental principle of matter’s unwavering presence takes center stage. Our comprehensive guide, aptly titled “Law of Conservation of Mass Worksheet Answers,” invites you on an enlightening journey through the intricacies of this scientific cornerstone.
This meticulously crafted resource delves into the depths of the Law of Conservation of Mass, unraveling its significance, exploring its practical applications, and addressing its exceptions. Prepare to embark on an intellectual adventure that will illuminate your understanding of matter’s unyielding nature.
Law of Conservation of Mass
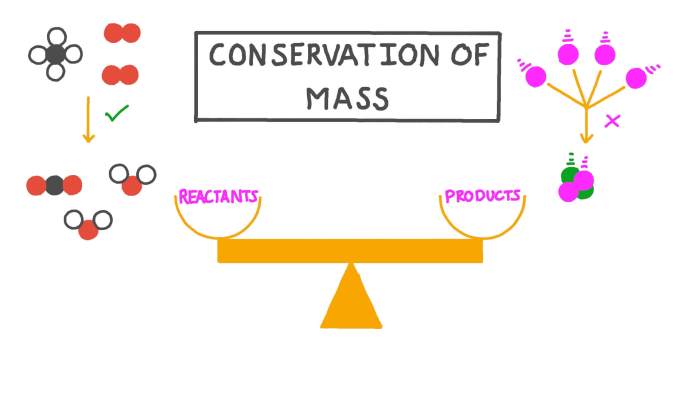
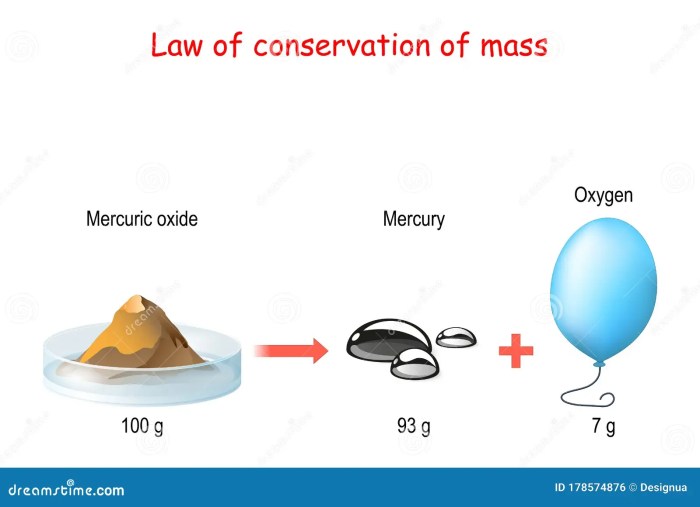
The law of conservation of mass states that the total mass of a closed system remains constant, regardless of changes in state or composition of the system. This means that matter cannot be created or destroyed, only transformed from one form to another.
The law of conservation of mass is one of the fundamental laws of chemistry and physics. It has been experimentally verified countless times and is considered to be one of the most important laws of science.
Significance of the Law of Conservation of Mass, Law of conservation of mass worksheet answers
The law of conservation of mass has several important implications. First, it means that the total amount of matter in the universe is constant. This is a fundamental principle of science and has important implications for our understanding of the universe.
Second, the law of conservation of mass can be used to balance chemical equations. A balanced chemical equation shows the reactants and products of a chemical reaction and the stoichiometric coefficients that balance the number of atoms of each element on both sides of the equation.
The law of conservation of mass ensures that the total mass of the reactants is equal to the total mass of the products.
Third, the law of conservation of mass can be used to solve problems involving chemical reactions. For example, if we know the mass of the reactants and the products of a reaction, we can use the law of conservation of mass to calculate the mass of any missing reactant or product.
Examples of the Law of Conservation of Mass in Action
- When a candle burns, the wax and oxygen in the air react to form carbon dioxide and water vapor. The total mass of the reactants is equal to the total mass of the products, demonstrating the law of conservation of mass.
- When a plant grows, it absorbs carbon dioxide and water from the air and soil. The plant uses these materials to build new plant tissue. The total mass of the plant increases, but the total mass of the carbon dioxide and water in the environment decreases.
This is because the carbon and oxygen atoms in the carbon dioxide and water are incorporated into the plant tissue, demonstrating the law of conservation of mass.
- When a chemical reaction takes place in a closed container, the total mass of the reactants is equal to the total mass of the products. This is because the container prevents any matter from entering or leaving the system, demonstrating the law of conservation of mass.
Commonly Asked Questions: Law Of Conservation Of Mass Worksheet Answers
What is the Law of Conservation of Mass?
The Law of Conservation of Mass states that matter cannot be created or destroyed, only transformed from one form to another.
How can I use the Law of Conservation of Mass to solve chemistry problems?
By applying the Law of Conservation of Mass, you can determine the mass of reactants and products in chemical reactions.
Are there any exceptions to the Law of Conservation of Mass?
Yes, there are a few exceptions, such as nuclear reactions and certain chemical reactions involving energy changes.