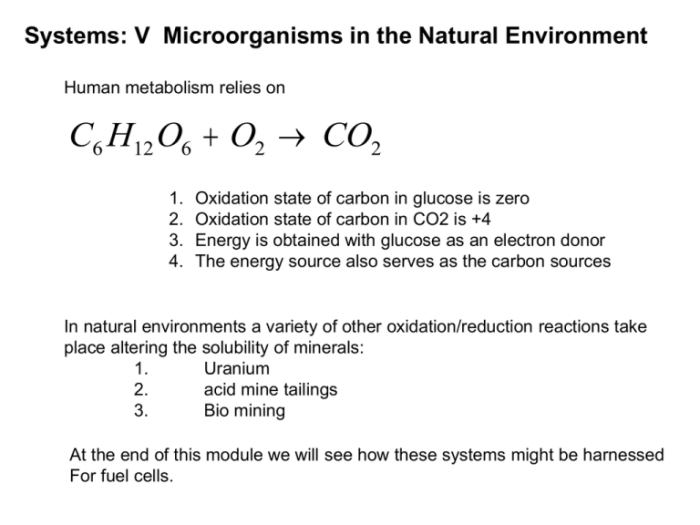
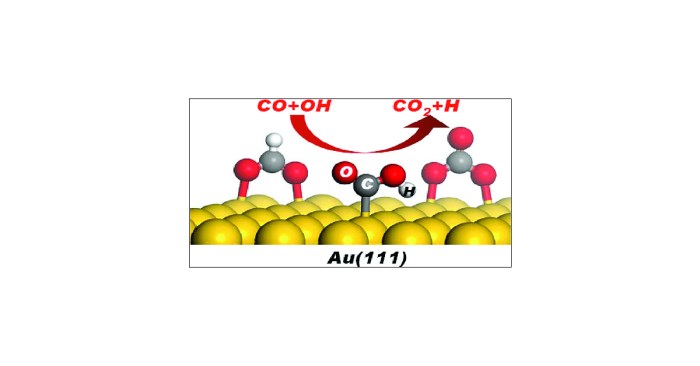
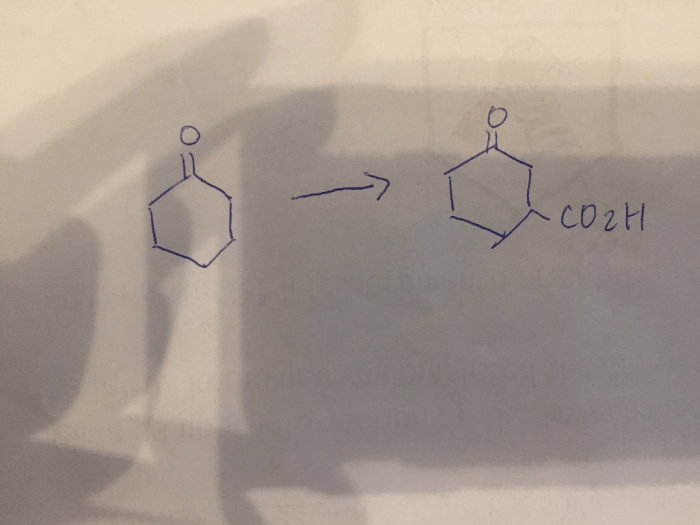Draw the structure of pyruvate at ph 7.4 – In the realm of biochemistry, understanding the structure of pyruvate at pH 7.4 holds immense significance. This intricate molecule plays a pivotal role in cellular metabolism, and its structure directly influences its function. Delving into the chemical intricacies of pyruvate at pH 7.4, this comprehensive overview explores its various forms, equilibrium dynamics, and biological implications.
The diverse forms of pyruvate at pH 7.4 arise from its unique chemical properties. The presence of a keto group and a carboxyl group allows pyruvate to exist in both keto and enol forms. At pH 7.4, the keto form predominates, accounting for approximately 99% of the total pyruvate pool.
However, the enol form, though less abundant, plays a crucial role in certain enzymatic reactions.
Pyruvate Structure at pH 7.4: Draw The Structure Of Pyruvate At Ph 7.4

Pyruvate, a key intermediate in cellular metabolism, exists in different forms at physiological pH 7.4. The predominant form is the enol form, which has a double bond between the carbonyl carbon and the adjacent carbon atom. A small percentage exists in the keto form, which has a carbonyl group on the terminal carbon atom.
The equilibrium between the enol and keto forms is pH-dependent, with the enol form being favored at neutral pH.
Factors Influencing Pyruvate Structure
pH plays a significant role in determining the structure of pyruvate. At low pH, the keto form predominates, while at higher pH, the enol form is more stable. Other factors that can influence the equilibrium between the two forms include temperature, ionic strength, and the presence of metal ions.
Biological Significance of Pyruvate Structure, Draw the structure of pyruvate at ph 7.4
The structure of pyruvate is crucial for its function in cellular metabolism. The enol form of pyruvate is the active form that can participate in metabolic reactions, such as the citric acid cycle and gluconeogenesis. The keto form is less reactive and serves as a storage form of pyruvate.
Experimental Methods for Studying Pyruvate Structure
Various experimental techniques are used to determine the structure of pyruvate at pH 7.4. These include nuclear magnetic resonance (NMR) spectroscopy, infrared spectroscopy, and X-ray crystallography. Each method provides different information about the molecular structure and dynamics of pyruvate.
Applications of Pyruvate Structure Knowledge
Understanding the structure of pyruvate has implications in medicine, biotechnology, and other fields. In medicine, it aids in understanding metabolic disorders and designing drugs that target pyruvate metabolism. In biotechnology, it helps in developing enzymes and biocatalysts that utilize pyruvate as a substrate or cofactor.
Further research on pyruvate structure could lead to advancements in these areas.
FAQ Summary
What is the predominant form of pyruvate at pH 7.4?
The keto form is the predominant form of pyruvate at pH 7.4, accounting for approximately 99% of the total pyruvate pool.
How does pH influence pyruvate structure?
pH significantly influences pyruvate structure by altering the equilibrium between the keto and enol forms. At higher pH values, the enol form becomes more prevalent.
What is the biological significance of pyruvate structure?
Pyruvate structure plays a crucial role in cellular metabolism, particularly in energy production and the synthesis of various biomolecules.